Partnerships for Drug Development
The CMTA works with the pharmaceutical, biotechnology and research service industries, along with non-profit research organizations and the National Institutes of Health.
These relationships are a critical part of the CMTA’s STAR initiative to rapidly deliver therapies to CMT patients.
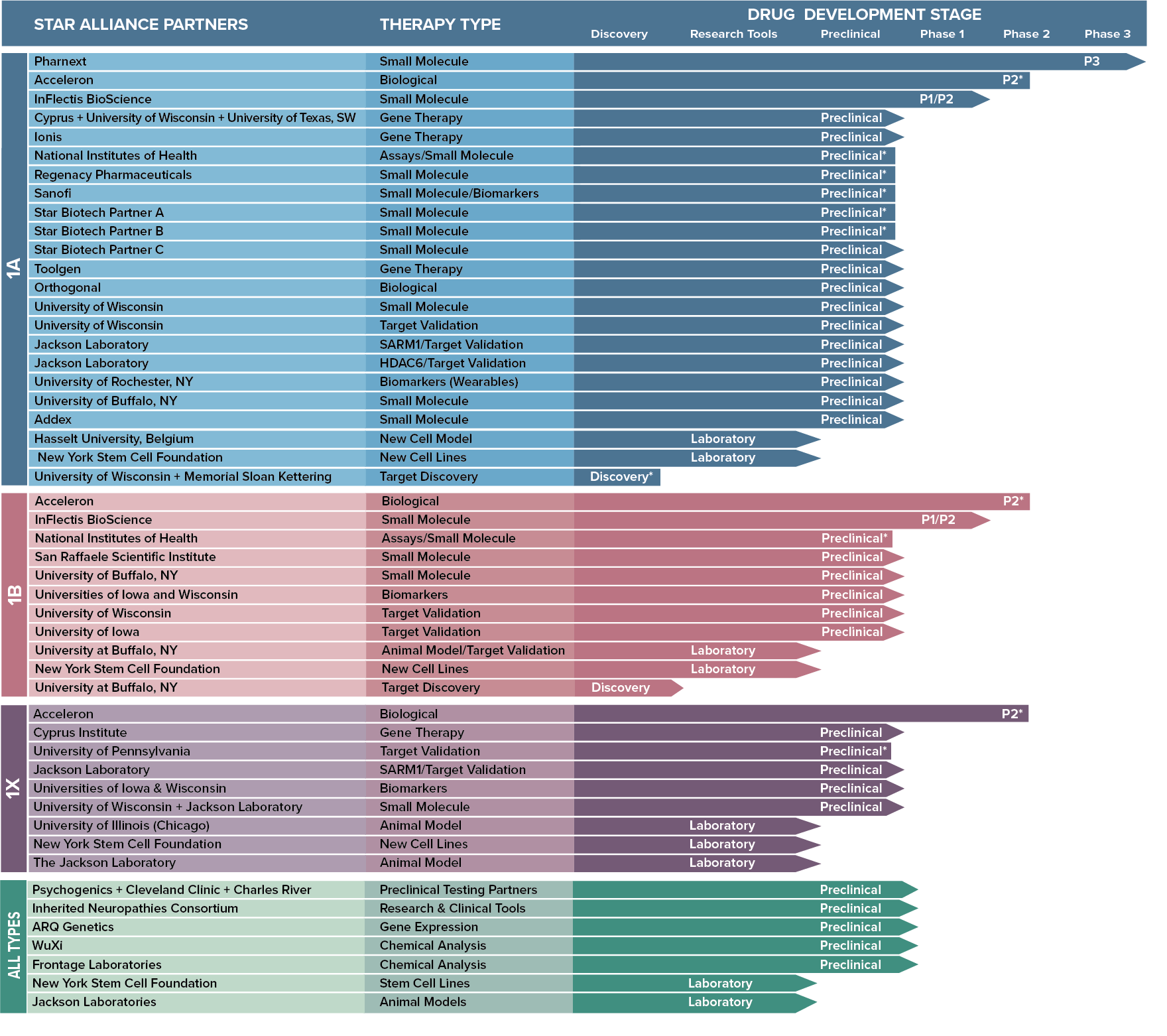
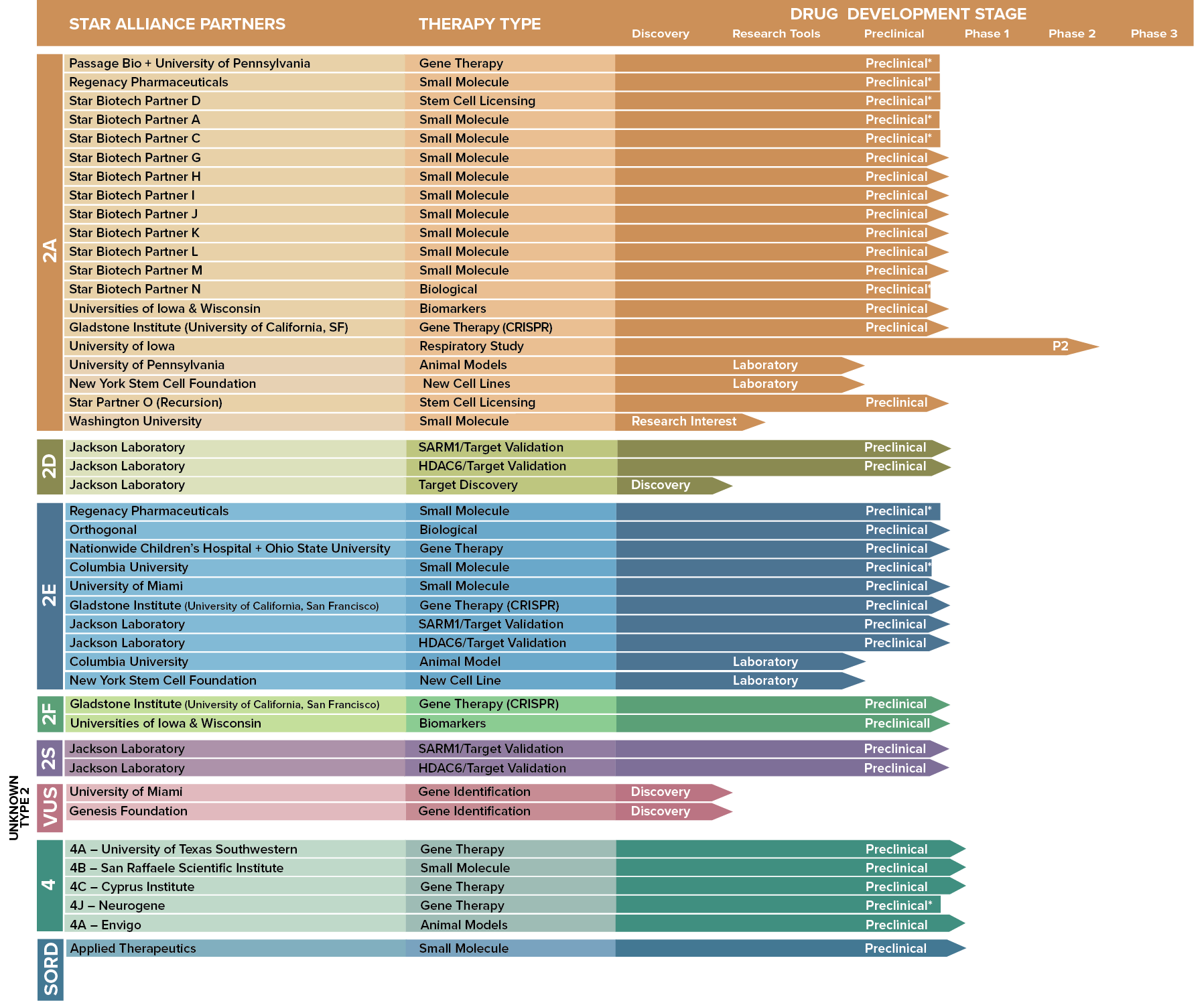
Since 2008, STAR has invested more than $18.5 million in funding to CMT research. Currently, the STAR Drug Development Pipeline involves more than 40 research partners participating in more than 50 research studies.
THE CMTA-STAR Drug Development Pipeline
Addex Therapeutics
 Addex Therapeutics is a clinical-stage pharmaceutical company pioneering allosteric modulation-based drug discovery and development. CMTA and Addex formed a collaboration to investigate a potential therapy for CMT type 1A (CMT1A). The primary goal of the collaboration was to evaluate the benefit of Addex’s proprietary positive allosteric modulator’s (PAM’s) targeting the gamma-Aminobutyric acid subtype B (GABAB) receptor in rodent models of CMT1A. The GABAB receptor has previously been shown to be instrumental in controlling the overexpression of Peripheral Myelin Protein-22 (PMP22) in a rat model of CMT1A. Elevated PMP22 is closely associated with the disabling peripheral neuropathy that accompanies CMT1A.
Addex Therapeutics is a clinical-stage pharmaceutical company pioneering allosteric modulation-based drug discovery and development. CMTA and Addex formed a collaboration to investigate a potential therapy for CMT type 1A (CMT1A). The primary goal of the collaboration was to evaluate the benefit of Addex’s proprietary positive allosteric modulator’s (PAM’s) targeting the gamma-Aminobutyric acid subtype B (GABAB) receptor in rodent models of CMT1A. The GABAB receptor has previously been shown to be instrumental in controlling the overexpression of Peripheral Myelin Protein-22 (PMP22) in a rat model of CMT1A. Elevated PMP22 is closely associated with the disabling peripheral neuropathy that accompanies CMT1A.
The research alliance with Addex concluded with joint study planning aimed at the chronic dosing of select GABAB PAM’s in rodent models of CMT1A, followed by detailed assessments aimed at measuring the improvement of key outcomes. These outcome measures include biomarkers, motor function, electrophysiology and peripheral nerve histology.
Acceleron Pharma
 Acceleron Pharma, a Cambridge, MA based biopharmaceutical company dedicated to developing medicines to treat serious and rare diseases, developed ACE-083, a therapeutic candidate designed to affect muscles to maximize growth and strength. Acceleron initially developed ACE-083 for disorders such as CMT and FSHD (Facioscapulohumeral Muscular Dystrophy). After Phase 2 Trials in both CMT and FSHD, Acceleron concluded studies.
Acceleron Pharma, a Cambridge, MA based biopharmaceutical company dedicated to developing medicines to treat serious and rare diseases, developed ACE-083, a therapeutic candidate designed to affect muscles to maximize growth and strength. Acceleron initially developed ACE-083 for disorders such as CMT and FSHD (Facioscapulohumeral Muscular Dystrophy). After Phase 2 Trials in both CMT and FSHD, Acceleron concluded studies.
Applied Therapeutics
 Applied Therapeutics, a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates against validated molecular targets in indications of high unmet medical need, and the CMTA announced a collaboration to investigate a potential therapy for a newly discovered type of the disease caused by a deficiency of the SORD gene.
Applied Therapeutics, a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates against validated molecular targets in indications of high unmet medical need, and the CMTA announced a collaboration to investigate a potential therapy for a newly discovered type of the disease caused by a deficiency of the SORD gene.
The primary goal of the collaboration is to help identify patients that may be eligible to participate in upcoming clinical trials and provide insight into clinical trial planning through the CMTA’s Patients as Partners in Research initiative. Through this collaboration, patients will be able to get free SORD testing by having a nurse come to their home or through their doctor’s office, and the patient perspective will be shared to help shape trials to come.
A team led by Dr. Stephan Züchner at the University of Miami discovered SORD. Züchner’s team, including Drs. Andrea Cortese, Grace Zhai, Adriana Rebelo and many others, found that mutations in the SORD gene cause an axonal form of CMT that is recessive. The newly discovered type is caused by a mutated SORD (sorbitol dehydrogenase) gene that raises sorbitol levels so high they cause nerve damage. Researchers found that treating fruit flies with a type of drug called an aldose reductase inhibitor, reduced their high levels of sorbitol to near normal. It is estimated that at least 3,000 –4,000 people in the United States and 4,000 patients in Europe, have this type of CMT, making it the most common recessive form of the disease.
AT-007 is an oral aldose reductase inhibitor in development for SORD. Because the drug is still in clinical trials, it’s called an “investigational drug.” AT-007 blocks the enzyme that precedes sorbitol dehydrogenase to prevent sorbitol from being formed in the body. In a recent pilot study in 8 SORD patients, AT-007 reduced sorbitol levels by 66% in the blood, with a range of individual patient reductions of 54%-75%. AT-007 has also been studied in healthy volunteers as well as adults and children with another rare disease called Galactosemia. Applied Therapeutics is planning to initiate a larger registrational study in SORD patients with sites in the US and Europe towards the end of this year. The hope is that the registrational trial will support approval of the first drug to treat SORD.
Genzyme (a Sanofi Company)
 In September 2014, the CMTA entered an alliance with Genzyme, a Sanofi company, to test their large libraries of molecules to identify those that dampen the known disease defect in CMT1A. A screening test capable of the automation required for this work was developed in a collaboration between the University of Wisconsin and the NIH. Following testing of close to 2 million molecules at Sanofi, a number of chemical classes were identified that suppress the overexpression of PMP22, a key protein implicated in the causation of CMT1A. Candidates from these chemicals were tested in animal models of the disease. In addition, the alliance expanded to the evaluation of additional molecules that emerged from other Sanofi Programs and represent an advanced state of drug design. Several of these drug prototypes have been tested in animal disease models of CMT1A. The CMTA Sanofi partnership also involved the development of important biomarkers for CMT1A.
In September 2014, the CMTA entered an alliance with Genzyme, a Sanofi company, to test their large libraries of molecules to identify those that dampen the known disease defect in CMT1A. A screening test capable of the automation required for this work was developed in a collaboration between the University of Wisconsin and the NIH. Following testing of close to 2 million molecules at Sanofi, a number of chemical classes were identified that suppress the overexpression of PMP22, a key protein implicated in the causation of CMT1A. Candidates from these chemicals were tested in animal models of the disease. In addition, the alliance expanded to the evaluation of additional molecules that emerged from other Sanofi Programs and represent an advanced state of drug design. Several of these drug prototypes have been tested in animal disease models of CMT1A. The CMTA Sanofi partnership also involved the development of important biomarkers for CMT1A.
InFlectis BioScience
 The CMTA entered into an Alliance Partnership with InFlectis BioScience, a clinical stage company committed to the development of innovative therapeutics harnessing the Integrated Stress Response (ISR) for the treatment of a broad range of diseases. The company plans to demonstrate the clinical effectiveness of its drug candidate IFB-088 for the treatment of Charcot-Marie-Tooth disease types 1A and 1B. In May 2018, the French National Agency for Medicines and Health Products Safety approved the Company’s Clinical Trial Application to begin a Phase 1 study of IFB-088 which will provide the safety data necessary for Phase 2 studies in CMT patients. The CMTA and InFlectis BioScience alliance team is collaborating on pre-clinical studies, clinical planning and understanding the impact of the disease on patients
The CMTA entered into an Alliance Partnership with InFlectis BioScience, a clinical stage company committed to the development of innovative therapeutics harnessing the Integrated Stress Response (ISR) for the treatment of a broad range of diseases. The company plans to demonstrate the clinical effectiveness of its drug candidate IFB-088 for the treatment of Charcot-Marie-Tooth disease types 1A and 1B. In May 2018, the French National Agency for Medicines and Health Products Safety approved the Company’s Clinical Trial Application to begin a Phase 1 study of IFB-088 which will provide the safety data necessary for Phase 2 studies in CMT patients. The CMTA and InFlectis BioScience alliance team is collaborating on pre-clinical studies, clinical planning and understanding the impact of the disease on patients
Ionis Pharmaceuticals
 In March of 2014, the CMTA entered an alliance with Ionis Pharmaceuticals (Ionis), a leading company in the development of Anti-Sense-Oligonucleotide (ASO) therapies. ASOs are short synthetic pieces of chemically modified nucleic acids (DNA or RNA) designed to bind to the RNA that codes for a protein. Once the ASO binds to the RNA it causes the destruction of the RNA by cellular enzymes, effectively leading to a decrease in the amount of targeted protein that is expressed. Ionis had previously demonstrated the ability to deliver an ASO to peripheral nerves and wanted to work with the CMTA to design a therapy that would decrease PMP22 protein levels in CMT1A patients. Specific ASOs were designed which proved effective in altering PMP22 RNA levels in two different rodent models of CMT1A. Importantly, this treatment also led to functional improvements in the animals. This groundbreaking work is now reported in the Journal of Clinical Investigation. The alliance team is now focused on the further design and testing of drug candidates that will be suitable for testing in patients.
In March of 2014, the CMTA entered an alliance with Ionis Pharmaceuticals (Ionis), a leading company in the development of Anti-Sense-Oligonucleotide (ASO) therapies. ASOs are short synthetic pieces of chemically modified nucleic acids (DNA or RNA) designed to bind to the RNA that codes for a protein. Once the ASO binds to the RNA it causes the destruction of the RNA by cellular enzymes, effectively leading to a decrease in the amount of targeted protein that is expressed. Ionis had previously demonstrated the ability to deliver an ASO to peripheral nerves and wanted to work with the CMTA to design a therapy that would decrease PMP22 protein levels in CMT1A patients. Specific ASOs were designed which proved effective in altering PMP22 RNA levels in two different rodent models of CMT1A. Importantly, this treatment also led to functional improvements in the animals. This groundbreaking work is now reported in the Journal of Clinical Investigation. The alliance team is now focused on the further design and testing of drug candidates that will be suitable for testing in patients.
Orthogonal Neuroscience
 Through a Preclinical Testing Alliance, Orthogonal has gained access to the CMTA’s service research providers and preclinical testing network. The CMTA provided bespoke support around selection of appropriate models, design of the experiments, data interpretation and guidance regarding what future experimentswould be needed to take the project forward. The CMTA also made introductions to key clinicians and thought leaders tofacilitate future clinical trials in CMT patients. The CMTA Preclinical Testing Alliance facilitated ORT247 testsin CMT1A (demyelinating) and 2E (axonopathy) mouse modelsand the results show an improvement in several disease endpoints.
Through a Preclinical Testing Alliance, Orthogonal has gained access to the CMTA’s service research providers and preclinical testing network. The CMTA provided bespoke support around selection of appropriate models, design of the experiments, data interpretation and guidance regarding what future experimentswould be needed to take the project forward. The CMTA also made introductions to key clinicians and thought leaders tofacilitate future clinical trials in CMT patients. The CMTA Preclinical Testing Alliance facilitated ORT247 testsin CMT1A (demyelinating) and 2E (axonopathy) mouse modelsand the results show an improvement in several disease endpoints.
Passage Bio
![]() Passage Bio, a genetic medicines company developing AAV-delivered gene therapies for the treatment of rare monogenic central nervous system (CNS) diseases, has licensed a sixth gene therapy development program under its research, collaboration and license agreement with the University of Pennsylvania and its Gene Therapy Program (GTP). The license is for the clinical development of a potential treatment for patients with Charcot-Marie-Tooth Neuropathy Type 2A (CMT2A). The Gene Therapy Program at Penn has developed AAV vectors and delivery methods to target the nerve cells that are affected in CMT2A, raising the possibility of slowing or preventing progression of the disease by tackling the underlying genetic cause. Passage Bio will develop this experimental therapy, designed to restore the normal function of the MFN2 gene, which is mutated in patients with CMT2A and looks forward to initiating a clinical trial in the near future.
Passage Bio, a genetic medicines company developing AAV-delivered gene therapies for the treatment of rare monogenic central nervous system (CNS) diseases, has licensed a sixth gene therapy development program under its research, collaboration and license agreement with the University of Pennsylvania and its Gene Therapy Program (GTP). The license is for the clinical development of a potential treatment for patients with Charcot-Marie-Tooth Neuropathy Type 2A (CMT2A). The Gene Therapy Program at Penn has developed AAV vectors and delivery methods to target the nerve cells that are affected in CMT2A, raising the possibility of slowing or preventing progression of the disease by tackling the underlying genetic cause. Passage Bio will develop this experimental therapy, designed to restore the normal function of the MFN2 gene, which is mutated in patients with CMT2A and looks forward to initiating a clinical trial in the near future.
Regenacy Pharmaceuticals
 The CMTA and Regenacy Pharmaceuticals, a clinical-stage biopharmaceutical company developing breakthrough treatments for diabetic and other peripheral neuropathies, engaged in a collaboration to validate the role of HDAC6 in multiple forms of Charcot-Marie-Tooth (CMT). Regenacy had an opportunity to expand on the groundbreaking work of Dr. Ludo van den Bosch at University of Leuven (published) and Dr. Andrew Grierson at University of Sheffield to show the role of HDAC6 in several forms of CMT. The collaboration focused on evaluating the efficacy of ricolinostat in animal models of CMT through the extensive preclinical testing capabilities the CMTA has assembled. Although Regenacy decided to conclude testing, the CMTA continues to explore the role of HDAC6 inhibitors.
The CMTA and Regenacy Pharmaceuticals, a clinical-stage biopharmaceutical company developing breakthrough treatments for diabetic and other peripheral neuropathies, engaged in a collaboration to validate the role of HDAC6 in multiple forms of Charcot-Marie-Tooth (CMT). Regenacy had an opportunity to expand on the groundbreaking work of Dr. Ludo van den Bosch at University of Leuven (published) and Dr. Andrew Grierson at University of Sheffield to show the role of HDAC6 in several forms of CMT. The collaboration focused on evaluating the efficacy of ricolinostat in animal models of CMT through the extensive preclinical testing capabilities the CMTA has assembled. Although Regenacy decided to conclude testing, the CMTA continues to explore the role of HDAC6 inhibitors.
Shift Pharmaceuticals
 Shift Pharmaceuticals, a privately held company that is developing antisense oligonucleotides (ASOs) to treat a variety of genetic disorders, and the Charcot-Marie-Tooth Association (CMTA) are collaborating to use ASOs to treat CMT1A, which is caused by a duplication in the PMP22 gene. ASOs are drugs that can alter RNA and reduce, restore or modify protein expression. Shift Pharmaceuticals will be taking advantage of the CMTA’s comprehensive suite of expert preclinical testing capabilities to evaluate the therapeutic potential of their drug candidate. The findings from this Alliance Partnership initiative will be critical in advancing research that will lead to clinical trials for CMT1A patients.
Shift Pharmaceuticals, a privately held company that is developing antisense oligonucleotides (ASOs) to treat a variety of genetic disorders, and the Charcot-Marie-Tooth Association (CMTA) are collaborating to use ASOs to treat CMT1A, which is caused by a duplication in the PMP22 gene. ASOs are drugs that can alter RNA and reduce, restore or modify protein expression. Shift Pharmaceuticals will be taking advantage of the CMTA’s comprehensive suite of expert preclinical testing capabilities to evaluate the therapeutic potential of their drug candidate. The findings from this Alliance Partnership initiative will be critical in advancing research that will lead to clinical trials for CMT1A patients.
Taysha Gene Therapies
 The CMTA has given a venture philanthropy grant to Taysha Gene Therapies (Nasdaq: TSHA), a patient-centric, pivotal-stage gene therapy company focused on developing and commercializing AAV-based gene therapies for the treatment of monogenic diseases of the central nervous system (CNS) in both rare and large patient populations, for undertaking a CMT4A gene therapy project. Taysha and the CMTA will jointly fund the project at UT Southwestern under the direction of Drs. Xin Chen and Steven Gray (co-principal investigators). The goal of the project is to directly deliver the GDAP1 gene with adeno-associated viral 9 (AAV9) to stop the neuropathy that happens in CMT4A. The findings from this project will be critical in advancing research that will potentially lead to clinical trials for CMT4A patients.
The CMTA has given a venture philanthropy grant to Taysha Gene Therapies (Nasdaq: TSHA), a patient-centric, pivotal-stage gene therapy company focused on developing and commercializing AAV-based gene therapies for the treatment of monogenic diseases of the central nervous system (CNS) in both rare and large patient populations, for undertaking a CMT4A gene therapy project. Taysha and the CMTA will jointly fund the project at UT Southwestern under the direction of Drs. Xin Chen and Steven Gray (co-principal investigators). The goal of the project is to directly deliver the GDAP1 gene with adeno-associated viral 9 (AAV9) to stop the neuropathy that happens in CMT4A. The findings from this project will be critical in advancing research that will potentially lead to clinical trials for CMT4A patients.
ToolGen
 ToolGen, a biotechnology company focusing on development of the genome editing platform technology and its applications in human therapeutics and agriculture, and the CMTA established a collaboration to investigate a potential therapy for CMT type 1A (CMT1A), using CRISPR gene editing technology.The primary goal of the collaboration is to facilitate the development of a novel gene-editing therapy for CMT1A that seeks to suppress overactivity of the causative disease gene, PMP22. The testing alliance with ToolGen involves access to the STAR Scientific and Clinical advisors of the CMTA, animal testing of the approach in the CMTA preclinical testing alliance network, and assistance with the planning of clinical trials in the USA.
ToolGen, a biotechnology company focusing on development of the genome editing platform technology and its applications in human therapeutics and agriculture, and the CMTA established a collaboration to investigate a potential therapy for CMT type 1A (CMT1A), using CRISPR gene editing technology.The primary goal of the collaboration is to facilitate the development of a novel gene-editing therapy for CMT1A that seeks to suppress overactivity of the causative disease gene, PMP22. The testing alliance with ToolGen involves access to the STAR Scientific and Clinical advisors of the CMTA, animal testing of the approach in the CMTA preclinical testing alliance network, and assistance with the planning of clinical trials in the USA.
New York Stem Cell Foundation
 In December 2014, the CMTA announced a collaboration with the New York Stem Cell Foundation (NYSCF) Research Institute to make human stem cell lines that represent the genetic disease defect for a collection of CMT disorders. These cell lines were derived from patient materials curated in a biobank at the University of Iowa and are now distributed by the NYSCF. The immediate use of these cell lines is to develop methods that allow these cells to mimic the Type 1 (Schwann Cell) or Type 2 (motor nerve) defects seen in CMT patients. The cell lines are now being incorporated in the therapy evaluation testing of alliance partners, which allows the CMT to conduct rapid drug testing in a patient-centric disease model at a very early stage. To date, 17 of these patient-derived stem cell lines have been successfully completed, representing six different CMT disorders. The NYSCF is making these available to researchers through its website and on-line ordering system as a means of broadly stimulating research on CMT using materials that directly represent cellular models of the disease condition.
In December 2014, the CMTA announced a collaboration with the New York Stem Cell Foundation (NYSCF) Research Institute to make human stem cell lines that represent the genetic disease defect for a collection of CMT disorders. These cell lines were derived from patient materials curated in a biobank at the University of Iowa and are now distributed by the NYSCF. The immediate use of these cell lines is to develop methods that allow these cells to mimic the Type 1 (Schwann Cell) or Type 2 (motor nerve) defects seen in CMT patients. The cell lines are now being incorporated in the therapy evaluation testing of alliance partners, which allows the CMT to conduct rapid drug testing in a patient-centric disease model at a very early stage. To date, 17 of these patient-derived stem cell lines have been successfully completed, representing six different CMT disorders. The NYSCF is making these available to researchers through its website and on-line ordering system as a means of broadly stimulating research on CMT using materials that directly represent cellular models of the disease condition.
The National Institutes of Health (U.S.A.)
 The National Institutes of Health (U.S.A.) is this nation’s premier medical research agency—supporting scientific studies that turn discovery into health solutions. The CMTA has had a close working relationship at the NIH with the National Center for Advancing Translational Sciences (NCATS), the National Institute of Neurological Disorders and Stroke (NINDS), and the NIH Office of Technology Transfer. This has led to sponsorship of several joint projects that aim to support CMTA alliance efforts.
The National Institutes of Health (U.S.A.) is this nation’s premier medical research agency—supporting scientific studies that turn discovery into health solutions. The CMTA has had a close working relationship at the NIH with the National Center for Advancing Translational Sciences (NCATS), the National Institute of Neurological Disorders and Stroke (NINDS), and the NIH Office of Technology Transfer. This has led to sponsorship of several joint projects that aim to support CMTA alliance efforts.
In addition, the NINDS provides funding and guidance to the Inherited Neuropathies Consortium, with support also provided by the CMTA. This consortium has assembled a large patient registry of CMT patients, is actively performing natural history studies of CMT diseases, and is evaluating measurement tools that may be useful in monitoring disease progression. The NINDS also provides research support to many of the STAR investigator laboratories.
A key element required for the formation of successful alliances is the ability to make available a “toolbox” of cellular and animal models for the evaluation of drug candidates along with the know-how required to expertly use these models. The CMTA has purposefully set out to make or acquire the tools needed for this work and has developed benchmarks for how to use them. This preclinical testing capability has been assembled via close collaboration with an expert network of service providers. Currently, with the aim of testing therapies at different stages of development, the preclinical toolbox is being applied to a number of alliance relationships that address different CMT disorders. Members of this STAR service network are as follows:
ARQ Genetics
 Providing real time PCR services for client sample analysis, ARQ Genetics is supporting CMTA efforts to analyze gene expression in animal disease models of CMT and quantitatively measure changes in molecular markers as predictors of response to potential therapeutics.
Providing real time PCR services for client sample analysis, ARQ Genetics is supporting CMTA efforts to analyze gene expression in animal disease models of CMT and quantitatively measure changes in molecular markers as predictors of response to potential therapeutics.
Charles River
 This global provider of contract animal research services is working with the CMTA to breed, cryopreserve and distribute rodent models of CMT that are used in the STAR alliance network.
This global provider of contract animal research services is working with the CMTA to breed, cryopreserve and distribute rodent models of CMT that are used in the STAR alliance network.
Horizon Discovery
 Horizon Discovery is a translational genomics company that develops and supplies patient-relevant drug discovery and diagnostic research tools, including contract research services. Horizon Discovery has worked closely with STAR investigators to design and create several new rodent models of CMT which are now entering into research and therapeutics discovery projects sponsored by the CMTA.
Horizon Discovery is a translational genomics company that develops and supplies patient-relevant drug discovery and diagnostic research tools, including contract research services. Horizon Discovery has worked closely with STAR investigators to design and create several new rodent models of CMT which are now entering into research and therapeutics discovery projects sponsored by the CMTA.
HumanFirst Therapeutics LLC
 HumanFirst Therapeutics (HFT) is a Research Consulting & Management Company that works with the CMTA to develop research strategies that lead to therapy development alliances. HFT has worked as a trusted partner with the CMTA since 2012 to provide the operational expertise and research direction needed to advance these alliance efforts to patients.
HumanFirst Therapeutics (HFT) is a Research Consulting & Management Company that works with the CMTA to develop research strategies that lead to therapy development alliances. HFT has worked as a trusted partner with the CMTA since 2012 to provide the operational expertise and research direction needed to advance these alliance efforts to patients.
The Jackson Laboratory
 The Jackson Laboratory is an independent, nonprofit organization focused on mammalian genetics research and is currently working with the CMTA to breed and distribute specialized mouse models of CMT in support of its research project efforts.
The Jackson Laboratory is an independent, nonprofit organization focused on mammalian genetics research and is currently working with the CMTA to breed and distribute specialized mouse models of CMT in support of its research project efforts.
PsychoGenics
 PsychoGenics is a leader in specialty preclinical contract research and drug discovery services, with a focus on neurodegenerative and psychiatric disorders. In 2013, the CMTA completed a master services agreement with PsychoGenics to provide biomarker and behavioral testing support to the STAR network. The company works closely with the CMTA to design and then execute preclinical drug testing studies in CMT animal models, as well as to characterize animal models destined for use in CMTA research efforts.
PsychoGenics is a leader in specialty preclinical contract research and drug discovery services, with a focus on neurodegenerative and psychiatric disorders. In 2013, the CMTA completed a master services agreement with PsychoGenics to provide biomarker and behavioral testing support to the STAR network. The company works closely with the CMTA to design and then execute preclinical drug testing studies in CMT animal models, as well as to characterize animal models destined for use in CMTA research efforts.
Renovo Neural
 Renovo Neural Inc. is a specialized preclinical research organization offering expert histology and electron microscopy services to the CMTA for use in both the characterization of new animal models of CMT and the evaluation of new therapeutics in support of its pharmaceutical alliances.
Renovo Neural Inc. is a specialized preclinical research organization offering expert histology and electron microscopy services to the CMTA for use in both the characterization of new animal models of CMT and the evaluation of new therapeutics in support of its pharmaceutical alliances.