Partnerships for Drug Development
The Charcot-Marie-Tooth Association (CMTA) works with the pharmaceutical, biotechnology and research service industries, along with non-profit research organizations and the National Institutes of Health.
These relationships are a critical part of CMTA’s STAR initiative to rapidly deliver therapies to CMT patients.
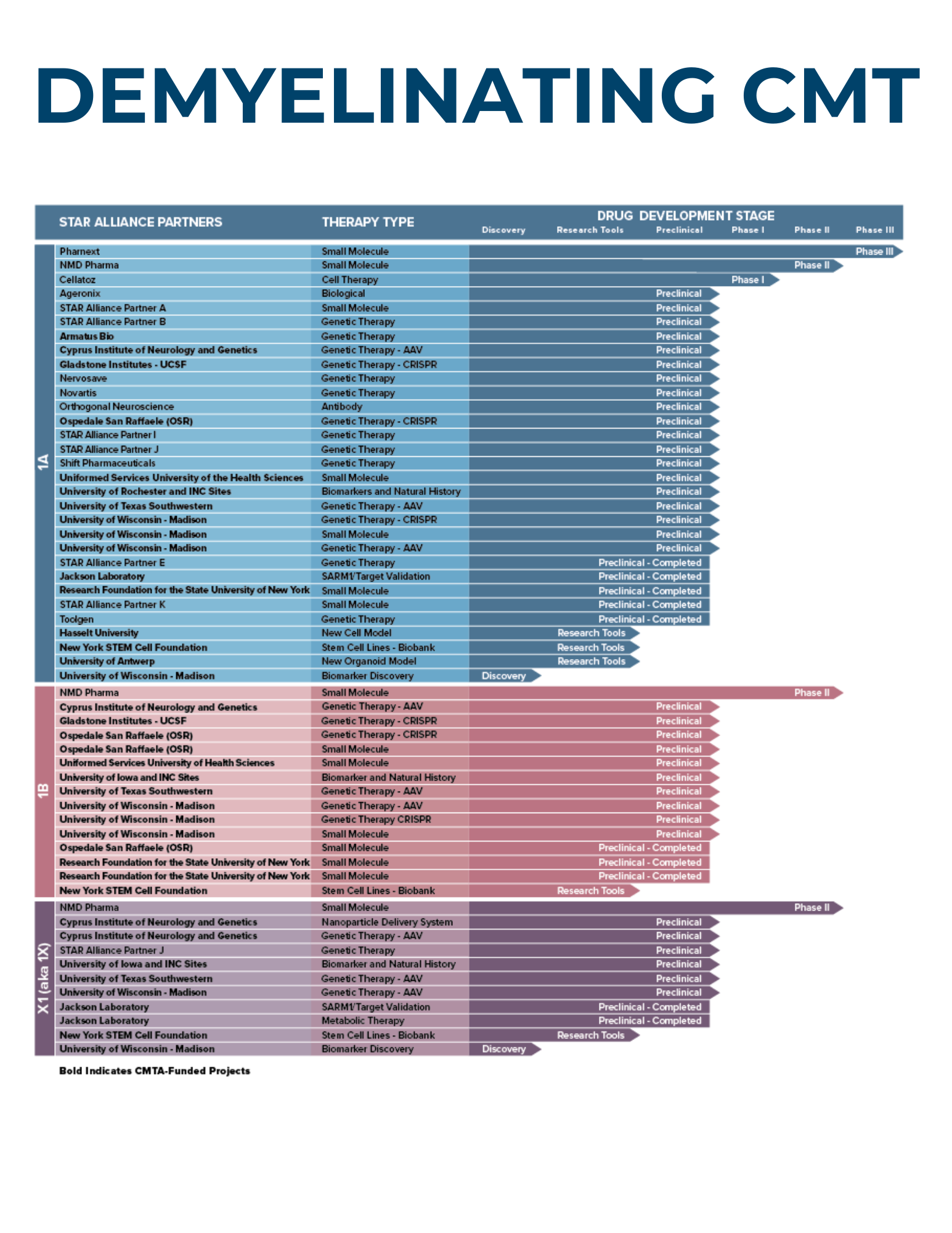
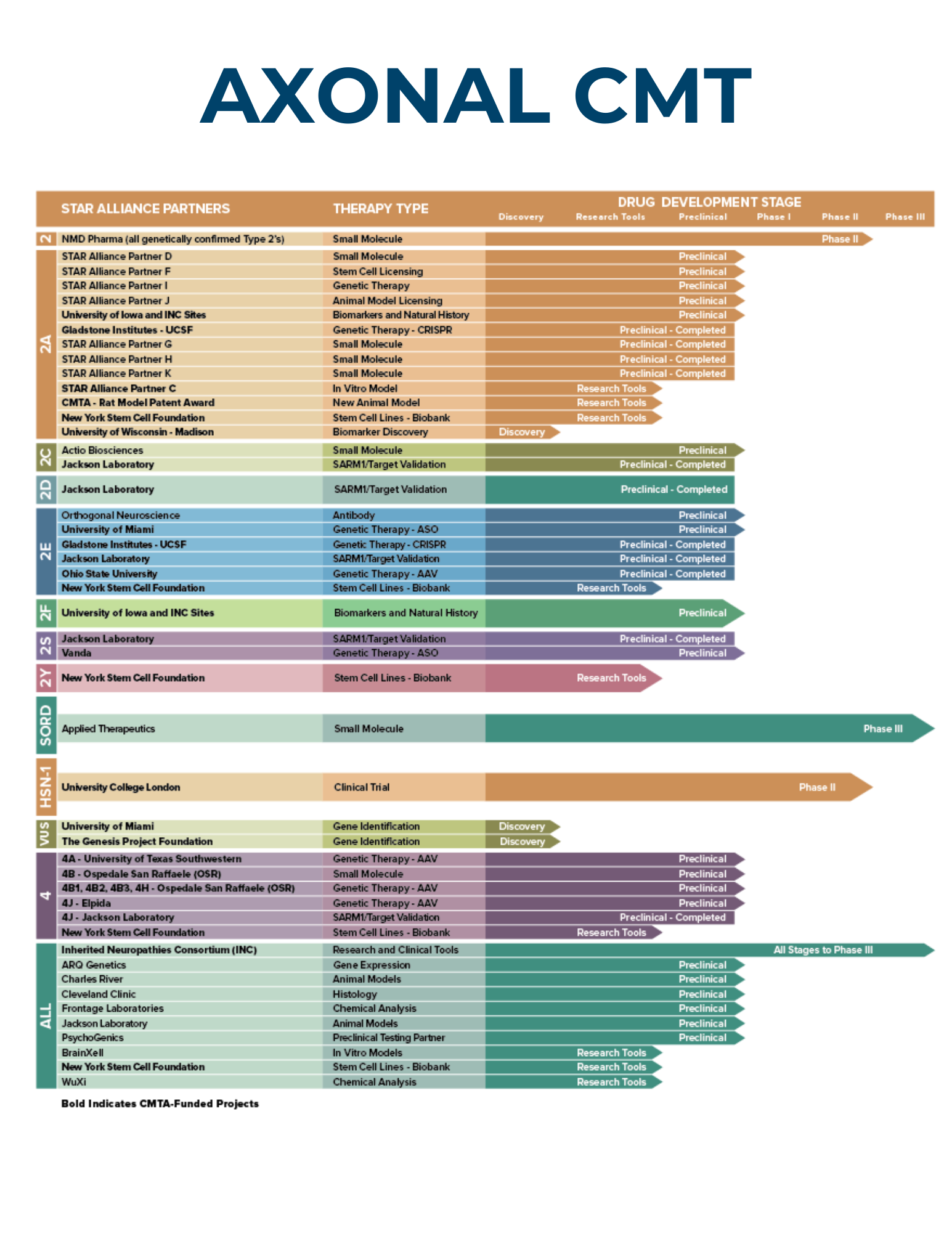
Since 2008, STAR has invested nearly $30 million to accelerate CMT research. Currently, the STAR Drug Development Pipeline involves more than 40 research partners participating in more than 50 research studies.
The CMTA-STAR Drug Development Pipeline

Actio Biosciences
Actio Biosciences was founded by leaders in genetics and drug development to push the boundaries of how we translate genetic insights into new medicines. Actio is more than a precision medicine company – they have a bold vision to take learnings in how to pinpoint targets and develop drugs for homogenous rare disease populations and leverage those learnings to identify underlying biology in heterogenous common disease populations. Through their unique approach, they aim to bring meaningful new therapeutics from one to many.
Read more
Ageronix
Ageronix is a privately held pharmaceutical company based in Geneva, Switzerland. With the innovative use of Alpha 1 Anti-trypsin, Ageronix stands at the forefront of Neurodegenerative Disease research. Ageronix was granted the Orphan Drug Designation (ODD) and the Rare Pediatric Disease (RPD) Designation by the FDA.
Read more
Alesta Therapeutics B.V.
Alesta Therapeutics is a pioneering company based in Leiden, Netherlands, dedicated to the development of first-in-class small molecules that target GCN2. Thier commitment to advanced, high-quality chemistry has led them to develop a clinical candidate with an extensive pre-GLP toxicology and CMC package.
Their work is focused on addressing a genetic subtype of Charcot-Marie-Tooth disease. To achieve this, they are combining their in-house expertise with the knowledge of global academic and industry-leading experts, such as CMTA. They are proud to collaborate with Professor Dr. Rob Burgess from the Jackson Laboratories, a renowned figure in this field.

ANANDA Devices
ANANDA Devices is a certified Women Owned SME, based in Montreal, Canada, that help companies comply with legislations to reduce animal experimentation by supplying platforms of human tissues-on-a-chip. Their high-throughput systems allow clients to accelerate drug development, toxicity screenings, disease modeling and more. Maintaining the goal of reducing animal testing, their devices allow for 100% human based models while increasing physiological relevance.
Read more
Applied Therapeutics
Applied Therapeutics, a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates against validated molecular targets in indications of high unmet medical need. In 2020, CMTA announced a collaboration to investigate a potential therapy for a newly discovered type of CMT caused by a mutation in the SORD gene. The primary goal of the collaboration is to help identify patients that may be eligible to participate in clinical trials and provide insight into clinical trial planning through CMTA’s Patients as Partners in Research initiative. Through this collaboration, patients will be able to get free CMT-SORD testing by having a nurse come to their home or through their doctor’s office, and the patient perspective will be shared to help shape trials to come. A team led by Dr. Stephan Züchner at the University of Miami discovered CMT-SORD. Züchner’s team, including Drs. Andrea Cortese, Grace Zhai, Adriana Rebelo and many others, found that mutations in the SORD gene cause an axonal form of CMT that is recessive. The newly discovered type is caused by a mutated SORD (sorbitol dehydrogenase) gene that raises sorbitol levels so high they cause nerve damage. Researchers found that treating fruit flies with a type of drug called an aldose reductase inhibitor, reduced their high levels of sorbitol to near normal. It is estimated that at least 3,000 –4,000 people in the United States and 4,000 patients in Europe, have this type of CMT, making it the most common recessive form of the disease. AT-007 is an oral aldose reductase inhibitor in development for SORD. Because the drug is still in clinical trials, it’s called an “investigational drug.” AT-007 blocks the enzyme that precedes sorbitol dehydrogenase to prevent sorbitol from being formed in the body. In a recent pilot study in 8 SORD patients, AT-007 reduced sorbitol levels by 66% in the blood, with a range of individual patient reductions of 54%-75%. AT-007 has also been studied in healthy volunteers as well as adults and children with another rare disease called Galactosemia. Applied Therapeutics is currently conducting a Phase 3 clinical trial in SORD patients with sites in the US and Europe. The hope is that the registrational trial will support approval of the first drug to treat SORD.
Read more
Armatus Bio
Armatus is harnessing the promise of RNA technology to raise expectations for clinical outcomes in challenging neuromuscular diseases with no therapeutic options today. Armatus is an emerging biotechnology company, based in Columbus, Ohio, that is advancing a unique gene therapy clinical candidate to target CMT1A, the most common form of the disease.
Read more
ARQ Genetics
Providing real time PCR services for client sample analysis, ARQ Genetics is supporting CMTA efforts to analyze gene expression in animal disease models of CMT and quantitatively measure changes in molecular markers as predictors of response to potential therapeutics.
Read more
Avicenna Biosciences
Avicenna Biosciences is a drug development company using machine learning-enhanced medicinal chemistry to accelerate the lead-to-candidate optimization process for small molecule drug development. Beyond their own assets, Avicenna collaborates with pharmaceutical and biotechnology companies to optimize their drug development campaigns and maximize risk-return profile.
Read more
BrainXell
BrainXell’s mission is to bring the most relevant neuronal cell models to researchers. BrainXell was founded in 2015 in conjunction with the Discovery to Product (D2P) program of University of Wisconsin- Madison and operates out of facility in University Research Park. The company is based on the proprietary technology of directed differentiation of human stem cells (including induced pluripotent stem cells) to highly enriched subclasses of neurons. BrainXell provides a range of high-purity, iPSC-derived human neurons for research and development with a focus on drug discovery. From motor neurons to cortical sub-types, they can provide specific models for both disease or normal drug screening. BrainXell also aims to develop stem cell therapy for neurological injuries and diseases through collaboration with pharmaceutical and healthcare industry.
Read more
Cellatoz Therapeutics
Cellatoz Therapeutics, Inc. is a biotech company developing innovative RMAT (regenerative medicine advanced therapy) programs and was established in August 2017 to develop innovative cell therapies aimed at solving unmet medical needs of intractable diseases and serious medical conditions. The company was founded by R&D experts to commercialize cell therapy products in Korea and they are working to create novel and unique cell therapy programs with strong capabilities in translational research and product development. Further, Cellatoz Therapeutics has proprietary technologies to differentiate Schwann cells from various source of cells and developed a regenerative cell therapy employing Schwann cells surrounding axons of neurons in peripheral nervous system to recover neuronal connections of damaged peripheral neurons due to accidents or diseases. The company is conduting clinical trails for CMT1A in Korea.
Read more
Charles River Laboratories
Charles River provides essential products and services to help pharmaceutical and biotechnology companies, government agencies, and leading academic institutions around the globe accelerate their research and drug development efforts. They deliver what their clients need to improve and expedite the discovery, early-stage development, and safe manufacture of new therapies for patients who need them. This global provider of contract animal research services is working with CMTA to breed, cryopreserve and distribute rodent models of CMT that are used in CMTA STAR’s preclinical testing infrastructure.
Read more
Cleveland Clinic
Cleveland Clinic is an American nonprofit academic medical center based in Cleveland, Ohio. Cleveland Clinic was at the forefront of modern medicine when its founders opened it as a multi-specialty group practice in 1921. In its first century, Cleveland Clinic has introduced many medical firsts, opened facilities around the world and is proud to be ranked among the top hospitals in the country. Now, 100 years later, the vision of the founders remains Cleveland Clinic’s mission: caring for life, researching for health, and educating those who serve. Cleveland Clinic works within CMTA’s preclinical testing infrastructure to provide histology services.
Read more
Elpida Therapeutics
Elpida’s mission aims to address the current and significant unmet medical needs of patients with ultra-rare diseases. Through leveraging scientific advancements and the now well-established safety and understanding of certain gene therapies, Elpida aims to put cures in reach of families and children who desperately and urgently need them. Elpida is conducting a Natural History Study in Charcot-Marie-Tooth Disease Type 4J. The aim of this study is to help clinical researchers gain a better understanding of the disease course in effected individuals that have not been well characterized in the clinic. The study will also help identify more people for clinical trials, inform the design of future clinical trials and identify therapeutic targets, potential biomarkers and endpoints to track treatment effects to be used in a planned gene therapy trial.
Read more
Frontage Laboratories
Frontage Laboratories, Inc. is a global pharmaceutical development organization (PDO) advancing drug discovery and development through integrated laboratory and clinical services. More than just a Contract Research Organization, they assume the role of drug developer, assisting clients in advancing hundreds of molecules through development to commercial launch in global markets. Their commitment to providing rigorous scientific expertise ensures the highest quality and compliance and allows them to deliver efficient and cost-effective development of new drugs.
Read more
Ionis Pharmaceuticals
Ionis Pharmaceuticals is a pioneer in RNA therapeutics. They have discovered and developed five currently marketed medicines for serious diseases, including breakthrough medicines for neurologic and cardiovascular diseases. With their groundbreaking science and technology, they have enhanced the profiles of their RNA-targeted medicines and unlocked new opportunities in emerging areas of genetic therapy. Historically, they have worked with other biotechnology companies as partners to deliver these medicines to patients who need them. In March of 2014, CMTA entered an alliance with Ionis Pharmaceuticals (Ionis), in the development of Anti-Sense-Oligonucleotide (ASO) therapies. Specific ASOs were designed which proved effective in altering PMP22 RNA levels in two different rodent models of CMT1A. Importantly, this treatment also led to functional improvements in the animals. These proof of concept stuides showed that lowering PMP22 leads to functional imprvements in CMT1A. While the Ionis therapeutic candidate was not suitable for use in patients, the pivotal work has attaced other companies into the space who currently have candadtes in preclinical development.
Read more
The Jackson Laboratory
The Jackson Laboratory is an independent, nonprofit organization focused on mammalian genetics research and works with CMTA to breed and distribute specialized mouse models of CMT in support of our research project efforts. Their team combines mouse genetics, human genomics, cell-based studies, and computational modeling to define the underlying biology of a broad spectrum of diseases. They leverage a unique combination of research, education and resources to achieve their mission: to discover precise genomic solutions for disease and EMPOWER the global biomedical community in its shared quest to improve human health.
Read more
Nervosave Therapeutics
Nervosave Therapeutics is developing a platform of local gene therapies that slow, stop and reverse a broad range of diseases involving peripheral nerves. Their first aim is to develop best in class gene therapy for CMT1A and then beyond for all CMT diseases. Their platform has game-changing potential in several devastating diseases with clear therapeutic needs such as neuralgias, diabetic peripheral neuropathy and other degenerative diseases involving nerves.
Read more
NMD Pharma A/S
NMD Pharma discovers and develops novel therapeutics for neuromuscular diseases. NMD Pharma has a unique experimental platform focusing on ion channel function and electrophysiology of skeletal muscle. They develop small molecule inhibitors of skeletal muscle-specific ClC-1 ion channels that have a promising therapeutic potential in a range of neuromuscular diseases in which muscle activation is failing, leading to muscle weakness and excessive fatigue that compromise the ability of patients to conduct essential human behavior. Their focus is on myasthenia gravis, spinal muscular atrophy, CMT, and sarcopenia. NMD’s Phase 2 SYNAPSE clinical trail for CMT launched in November 2024 with lead candidate NMD670 and is open to patients with Type 1 and Type 2 CMT.
Read more
Novartis
Novartis is an innovative medicines company. Every day, they work to reimagine medicine to improve and extend people’s lives so that patients, healthcare professionals and societies are empowered in the face of serious disease. Specific to neuroscience, their ambition is to create a transformational impact for people living with severe neurological conditions and their caregivers by discovering, developing and delivering innovative medicines that change the course of disease progression. In 2023, Novartis acquired the DTx FALCON platform and lead therpeutic candidtae DTx-1252 for the treatment of CMT1A.
Read more
NY Stem Cell Foundation
The mission of The New York Stem Cell Foundation (NYSCF) is to accelerate cures for the major diseases of our time through stem cell research. Working across many diseases and disciplines, the NYSCF Research Institute enables scientists to take on the major diseases of our time through stem cell research, leading technological and biological innovations that accelerate therapies. In December 2014, CMTA announced a collaboration with the NYSCF Research Institute to make human stem cell lines that represent the genetic disease defect for a collection of CMT subtypes. These cell lines were derived from patient materials curated through CMTA clinical Centers of Excellence and are now distributed by NYSCF. The immediate use of these cell lines is to develop methods that allow cells to mimic the Type 1 (Schwann cell) or Type 2 (motor nerve) defects seen in CMT patients. The cell lines are being used in therapy evaluation testing by CMTA Alliance Partners, allowing rapid therapeutic candidate testing in patient-centric disease models at a very early stage. Over 37 partners have so far made use of this world-leading CMT biobank.
Read more
Orthogonal Neuroscience
Orthogonal Neuroscience Inc. is developing treatments for CMT. Their lead candidate, ORT-247, is a monoclonal antibody that targets EphA4, which has shown promising results in preclinical models of CMT. Through a CMTA Preclinical Testing Alliance, Orthogonal has gained access to CMTA’s service research providers and network. CMTA provided bespoke support around selection of appropriate models, design of the experiments, data interpretation and guidance regarding what future experiments would be needed to take the project forward. CMTA also made introductions to key clinicians and thought leaders to facilitate future clinical trials in CMT patients. CMTA Preclinical Testing Alliance facilitated ORT247 tests in CMT1A (demyelinating) and 2E (axonopathy) CMT models and the results show an improvement in several disease endpoints.
Read more
PsychoGenics
PsychoGenics has established itself as a reputable partner in preclinical research for neurodegenerative disorders. Their depth of expertise in preclinical Peripheral Nervous System studies combined with a diverse portfolio of rodent disease models positions them to champion the development of groundbreaking treatments and reduce the attrition rate in clinical development. In 2013, CMTA completed a master services agreement with PsychoGenics to provide biomarker and behavioral testing support to the STAR Preclinical Testing Network. The company works closely with CMTA to design and then execute preclinicaltherapeutic testing studies in CMT animal models, as well as to characterize animal models destined for use in CMTA research efforts.
Read more
Shift Pharma
Shift Pharmaceuticals, a privately held company developing antisense oligonucleotides (ASOs) as therapeutic candidates for a variety of genetic disorders. Rather than alleviating certain disease side effects, these molecules “SHIFT” the body’s natural protein production back into alignment effectively addressing the core cause of the disease. Shift are investigating the use of these ASOs to treat CMT1A, which is caused by a duplication in the PMP22 gene. ASOs can alter RNA and reduce, restore or modify protein expression.
Read more
Vanda Pharmaceuticals
Vanda is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies and medicines to address high unmet medical needs and improve the lives of patients. They use new technologies, including genetics and genomics, to inform their drug discovery, clinical trials, and commercial positioning of their compounds. They are working to advance the use of novel approaches to deliver these new medicines to patients. Vanda have a antisense oligonucleotides (ASO) therpeutic candidate, VCA-894A, for CMT2S.
Read more
WuXi Biologics
WuXi Biologics is a leading global Contract Research, Development and Manufacturing Organization (CRDMO) offering end-to-end solutions that enable partners to discover, develop and manufacture biologics – from concept to commercialization – for the benefit of patients worldwide. WuXi Biologics leverages its technologies and expertise to provide customers with efficient and cost-effective biologics discovery, development and manufacturing solutions. Their mission is to accelerate and transform the discovery, development and manufacturing of biologics through a comprehensive open-access platform, enabling thier global healthcare partners and benefiting patients worldwide.
Read moreLast Updated: December 13, 2024