Page 8 - 2021 Spring CMTA Report - Special Research Edition
P. 8
AXON DEGENERATION CMTA FUNDS STUDY USING TWO
There are several genes involved in axon degeneration. Most notable ALREADY-APPROVED DRUGS TO
is one called SARM1. The SARM1 gene codes for a protein that functions
as an enzyme, affecting the levels of an important metabolite (NAD+) TREAT CMT1B
necessary for certain chemical processes in the body. So, what does this
mean for a patient dealing with loss of neuromuscular functioning?
All nerve cells have axons whose proper functioning is essential in
signaling muscles to contract. Axons are vulnerable to degeneration
due to several destructive injury-induced triggers. In some types of
neuropathy, a disease-induced (CMT) injury to the nerves causes
inflammation, activating SARM1, which reduces the levels of axonal
NAD+, and causes axonal degeneration.
Inhibiting the activation of SARM1 has the potential of preventing this
cascade of events from happening. Several companies are working to
develop compounds that inhibit SARM1, and it is thought that this will
prove to be a successful therapeutic for blocking injury-induced axonal
degeneration pathways.
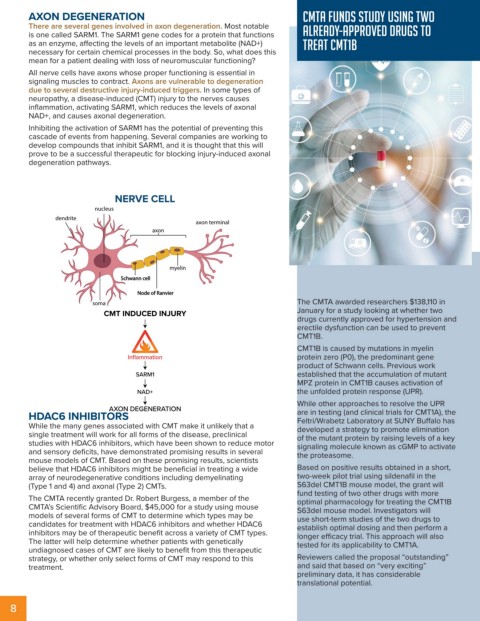
NERVE CELL
Schwann cell
Node of Ranvier
The CMTA awarded researchers $138,110 in
CMT INDUCED INJURY January for a study looking at whether two
drugs currently approved for hypertension and
erectile dysfunction can be used to prevent
CMT1B.
CMT1B is caused by mutations in myelin
Inflammation protein zero (P0), the predominant gene
product of Schwann cells. Previous work
SARM1 established that the accumulation of mutant
MPZ protein in CMT1B causes activation of
NAD+ the unfolded protein response (UPR).
While other approaches to resolve the UPR
AXON DEGENERATION are in testing (and clinical trials for CMT1A), the
HDAC6 INHIBITORS Feltri/Wrabetz Laboratory at SUNY Buffalo has
While the many genes associated with CMT make it unlikely that a developed a strategy to promote elimination
single treatment will work for all forms of the disease, preclinical of the mutant protein by raising levels of a key
studies with HDAC6 inhibitors, which have been shown to reduce motor signaling molecule known as cGMP to activate
and sensory deficits, have demonstrated promising results in several the proteasome.
mouse models of CMT. Based on these promising results, scientists
believe that HDAC6 inhibitors might be beneficial in treating a wide Based on positive results obtained in a short,
array of neurodegenerative conditions including demyelinating two-week pilot trial using sildenafil in the
(Type 1 and 4) and axonal (Type 2) CMTs. S63del CMT1B mouse model, the grant will
The CMTA recently granted Dr. Robert Burgess, a member of the fund testing of two other drugs with more
optimal pharmacology for treating the CMT1B
CMTA’s Scientific Advisory Board, $45,000 for a study using mouse S63del mouse model. Investigators will
models of several forms of CMT to determine which types may be use short-term studies of the two drugs to
candidates for treatment with HDAC6 inhibitors and whether HDAC6 establish optimal dosing and then perform a
inhibitors may be of therapeutic benefit across a variety of CMT types. longer efficacy trial. This approach will also
The latter will help determine whether patients with genetically tested for its applicability to CMT1A.
undiagnosed cases of CMT are likely to benefit from this therapeutic
strategy, or whether only select forms of CMT may respond to this Reviewers called the proposal “outstanding”
treatment. and said that based on “very exciting”
preliminary data, it has considerable
translational potential.
8